Lipids Are Soluble In What
Lipids
The lipids are a large and diverse grouping of naturally occurring organic compounds that are related by their solubility in nonpolar organic solvents (e.g. ether, chloroform, acetone & benzene) and general insolubility in h2o. At that place is groovy structural variety among the lipids, as will be demonstrated in the following sections. Y'all may click on a topic listed below, or proceed page by page.
Fatty Acids
Soaps and Detergents
Fats and Oils
Waxes
Phospholipids
Eicosonoids
Terpenes
Steroids
Lipid Soluble Vitamins
Biosynthetic Pathways
Fats, Oils, Waxes & Phospholipids
1. Fatty Acids
The common feature of these lipids is that they are all esters of moderate to long chain fatty acids. Acid or base-catalyzed hydrolysis yields the component fat acid, some examples of which are given in the following table, together with the alcohol component of the lipid. These long-chain carboxylic acids are mostly referred to by their mutual names, which in most cases reflect their sources. Natural fatty acids may be saturated or unsaturated, and equally the following data bespeak, the saturated acids accept college melting points than unsaturated acids of corresponding size. The double bonds in the unsaturated compounds listed on the right are all cis (or Z).
|
| ||||||||||||||||||||||||||||||||||||||||||
The higher melting points of the saturated fatty acids reverberate the uniform rod-similar shape of their molecules. The cis-double bond(south) in the unsaturated fatty acids introduce a kink in their shape, which makes it more difficult to pack their molecules together in a stable repeating assortment or crystalline lattice. The trans-double bail isomer of oleic acid, known as elaidic acid, has a linear shape and a melting indicate of 45 ºC (32 ºC higher than its cis isomer). The shapes of stearic and oleic acids are displayed in the models below. You may examine models of these compounds by clicking on the desired model picture .
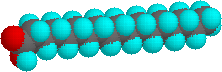 |  | |
|---|---|---|
| stearic acid | oleic acid |
Two polyunsaturated fatty acids, linoleic and linolenic, are designated "essential" considering their absenteeism in the human being diet has been associated with wellness problems, such equally scaley peel, stunted growth and increased aridity. These acids are also precursors to the prostaglandins, a family of physiologically stiff lipids present in infinitesimal amounts in well-nigh body tissues.
Because of their enhanced acerbity, carboxylic acids react with bases to form ionic salts, every bit shown in the following equations. In the case of alkali metal hydroxides and simple amines (or ammonia) the resulting salts have pronounced ionic graphic symbol and are ordinarily soluble in water. Heavy metals such as silvery, mercury and lead form salts having more covalent character (tertiary example), and the water solubility is reduced, particularly for acids composed of four or more carbon atoms.
| RCO2H | + | NaHCOiii |  | RCO2 (–) Na(+) + COii + HiiO |
| RCO2H | + | (CHiii)3Northward: |  | RCOii (–) (CH3)3NH(+) |
| RCO2H | + | AgOH |  | RCO2 δ(–) Agδ(+) + HtwoO |
Unusual Fatty Acids
Nature has constructed a remarkable multifariousness of fatty acrid derivatives. To come across some of these compounds Click Here.
ii. Soaps and Detergents
Carboxylic acids and salts having alkyl chains longer than eight carbons showroom unusual behavior in water due to the presence of both hydrophilic (CO2) and hydrophobic (alkyl) regions in the aforementioned molecule. Such molecules are termed amphiphilic (Gk. amphi = both) or amphipathic. 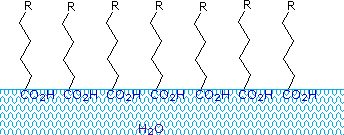 Fat acids made upward of ten or more carbon atoms are nearly insoluble in water, and because of their lower density, float on the surface when mixed with water. Dissimilar paraffin or other alkanes, which tend to puddle on the waters surface, these fatty acids spread evenly over an extended water surface, eventually forming a monomolecular layer in which the polar carboxyl groups are hydrogen bonded at the water interface, and the hydrocarbon chains are aligned together away from the h2o. This behavior is illustrated in the diagram on the correct. Substances that accumulate at water surfaces and alter the surface properties are called surfactants.
Fat acids made upward of ten or more carbon atoms are nearly insoluble in water, and because of their lower density, float on the surface when mixed with water. Dissimilar paraffin or other alkanes, which tend to puddle on the waters surface, these fatty acids spread evenly over an extended water surface, eventually forming a monomolecular layer in which the polar carboxyl groups are hydrogen bonded at the water interface, and the hydrocarbon chains are aligned together away from the h2o. This behavior is illustrated in the diagram on the correct. Substances that accumulate at water surfaces and alter the surface properties are called surfactants.
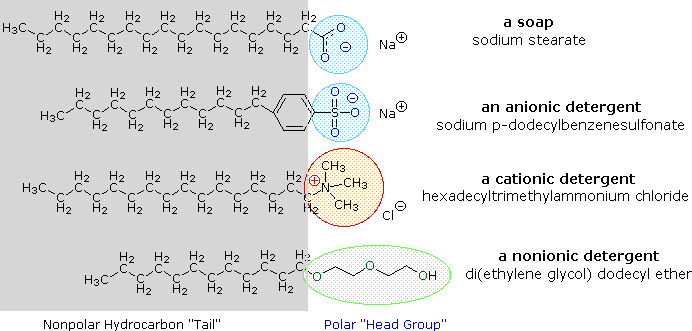
Brine metal salts of fat acids are more soluble in h2o than the acids themselves, and the amphiphilic character of these substances also brand them stiff surfactants. The most common examples of such compounds are soaps and detergents, iv of which are shown below. Notation that each of these molecules has a nonpolar hydrocarbon chain, the "tail", and a polar (often ionic) "head grouping". The use of such compounds equally cleaning agents is facilitated by their surfactant character, which lowers the surface tension of water, allowing it to penetrate and wet a variety of materials.
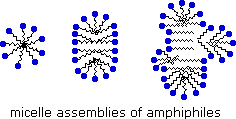
Very small amounts of these surfactants dissolve in water to give a random dispersion of solute molecules. Notwithstanding, when the concentration is increased an interesting change occurs. The surfactant molecules reversibly get together into polymolecular aggregates called micelles. By gathering the hydrophobic bondage together in the center of the micelle, disruption of the hydrogen bonded structure of liquid water is minimized, and the polar head groups extend into the surrounding h2o where they participate in hydrogen bonding. These micelles are often spherical in shape, but may also assume cylindrical and branched forms, as illustrated on the right. Here the polar head group is designated by a blue circumvolve, and the nonpolar tail is a zig-zag blackness line.
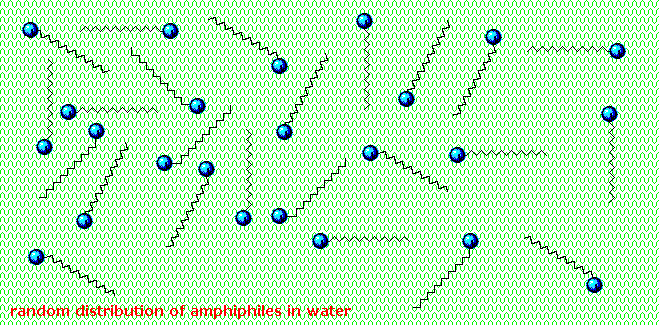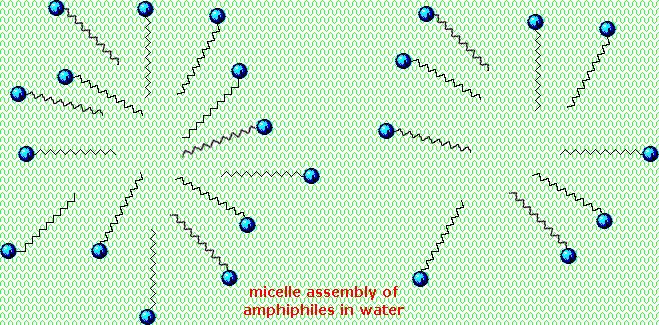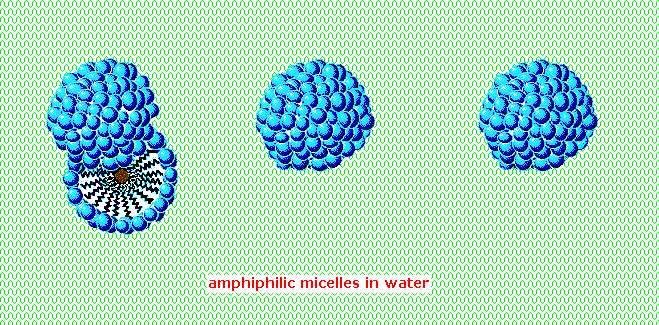
An blithe brandish of micelle formation is presented below. Observe the brownish material in the centre of the iii-dimensional drawing on the left. This illustrates a second important factor contributing to the use of these amphiphiles as cleaning agents. Micelles are able to encapsulate nonpolar substances such equally grease within their hydrophobic heart, and thus solubilize information technology so information technology is removed with the launder h2o. Since the micelles of anionic amphiphiles have a negatively charged surface, they repel 1 another and the nonpolar dirt is effectively emulsified. To summarize, the presence of a lather or a detergent in h2o facilitates the wetting of all parts of the object to be cleaned, and removes water-insoluble dirt by incorporation in micelles. If the animation has stopped, it may be restarted by clicking on it.
The oldest amphiphilic cleaning amanuensis known to humans is soap. Lather is manufactured by the base-catalyzed hydrolysis (saponification) of brute fat (see below). Earlier sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. Soft potassium soaps were then converted to the harder sodium soaps by washing with salt solution. The importance of lather to human being civilization is documented by history, only some problems associated with its use accept been recognized. I of these is caused past the weak acerbity (pKa ca. iv.ix) of the fatty acids. Solutions of brine metal soaps are slightly alkaline (pH eight to 9) due to hydrolysis. If the pH of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and course a scum. A second problem is caused past the presence of calcium and magnesium salts in the water supply (difficult water). These divalent cations cause aggregation of the micelles, which and then eolith as a dirty scum.
These bug have been alleviated by the development of synthetic amphiphiles called detergents (or syndets). By using a much stronger acrid for the polar caput group, water solutions of the amphiphile are less sensitive to pH changes. Also the sulfonate functions used for virtually all anionic detergents confer greater solubility on micelles incorporating the element of group i earth cations found in difficult h2o. Variations on the amphiphile theme have led to the development of other classes, such as the cationic and nonionic detergents shown higher up. Cationic detergents often exhibit germicidal properties, and their ability to change surface pH has made them useful as fabric softeners and hair conditioners. These versatile chemic "tools" have dramatically transformed the household and personal care cleaning product markets over the past fifty years.
iii. Fats and Oils
The triesters of fatty acids with glycerol (i,2,3-trihydroxypropane) compose the grade of lipids known as fats and oils. These triglycerides (or triacylglycerols) are found in both plants and animals, and etch 1 of the major nutrient groups of our diet. Triglycerides that are solid or semisolid at room temperature are classified as fats, and occur predominantly in animals. Those triglycerides that are liquid are called oils and originate chiefly in plants, although triglycerides from fish are besides largely oils. Some examples of the composition of triglycerides from diverse sources are given in the following tabular array.
| Saturated Acids (%) | Unsaturated Acids (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Source | C10 & less | C12 lauric | Cxiv myristic | Csixteen palmitic | C18 stearic | C18 oleic | C18 linoleic | C18 unsaturated |
| Animal Fats | ||||||||
| butter | fifteen | 2 | 11 | 30 | 9 | 27 | 4 | 1 |
| lard | - | - | 1 | 27 | fifteen | 48 | 6 | 2 |
| man fat | - | 1 | three | 25 | 8 | 46 | ten | 3 |
| herring oil | - | - | vii | 12 | 1 | two | 20 | 52 |
| Establish Oils | ||||||||
| coconut | - | 50 | 18 | eight | 2 | 6 | i | - |
| corn | - | - | 1 | 10 | 3 | fifty | 34 | - |
| olive | - | - | - | vii | 2 | 85 | 5 | - |
| palm | - | - | ii | 41 | 5 | 43 | 7 | - |
| peanut | - | - | - | 8 | three | 56 | 26 | 7 |
| safflower | - | - | - | 3 | iii | 19 | 76 | - |
As might be expected from the properties of the fat acids, fats have a predominance of saturated fatty acids, and oils are composed largely of unsaturated acids. Thus, the melting points of triglycerides reflect their composition, equally shown past the post-obit examples. Natural mixed triglycerides accept somewhat lower melting points, the melting betoken of lard being near 30 º C, whereas olive oil melts near -6 º C. Since fats are valued over oils by some Northern European and Northward American populations, vegetable oils are extensively converted to solid triglycerides (e.g. Crisco) by partial hydrogenation of their unsaturated components. Some of the remaining double bonds are isomerized (to trans) in this operation. These saturated and trans-fat acid glycerides in the nutrition have been linked to long-term wellness issues such as atherosclerosis.

Triglycerides having 3 identical acyl chains, such equally tristearin and triolein (above), are called "uncomplicated", while those composed of unlike acyl bondage are called "mixed". If the acyl chains at the end hydroxyl groups (ane & three) of glycerol are different, the center carbon becomes a chiral middle and enantiomeric configurations must be recognized.
The hydrogenation of vegetable oils to produce semisolid products has had unintended consequences. Although the hydrogenation imparts desirable features such equally spreadability, texture, "oral cavity feel," and increased shelf life to naturally liquid vegetable oils, it introduces some serious health problems. These occur when the cis-double bonds in the fatty acid chains are not completely saturated in the hydrogenation process. The catalysts used to result the addition of hydrogen isomerize the remaining double bonds to their trans configuration. These unnatural trans-fats appear to to exist associated with increased heart disease, cancer, diabetes and obesity, equally well equally allowed response and reproductive problems.
4. Waxes
Waxes are esters of fat acids with long chain monohydric alcohols (one hydroxyl group). Natural waxes are often mixtures of such esters, and may besides contain hydrocarbons. The formulas for three well known waxes are given below, with the carboxylic acid moiety colored red and the booze colored blueish.
| spermaceti | beeswax | carnuba wax | ||
|---|---|---|---|---|
| CH3(CH2)14CO2 -(CHii)15CHthree | CH3(CH2)24CO2 -(CH2)29CHiii | CH3(CH2)30COii -(CH2)33CHthree |
Waxes are widely distributed in nature. The leaves and fruits of many plants have waxy coatings, which may protect them from dehydration and pocket-sized predators. The feathers of birds and the fur of some animals have similar coatings which serve every bit a water repellent. Carnuba wax is valued for its toughness and water resistance.
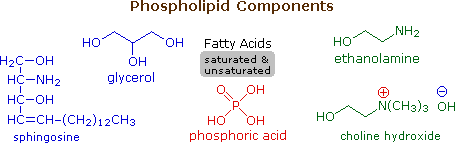
5. Phospholipids
Phospholipids are the main constituents of prison cell membranes. They resemble the triglycerides in being ester or amide derivatives of glycerol or sphingosine with fatty acids and phosphoric acid. The phosphate moiety of the resulting phosphatidic acid is farther esterified with ethanolamine, choline or serine in the phospholipid itself. The post-obit diagram shows the structures of some of these components. Clicking on the diagram volition change it to display structures for two representative phospholipids. Note that the fatty acid components (R & R') may be saturated or unsaturated.
To see a model of a phospholipid Click Here.
Equally ionic amphiphiles, phospholipids aggregate or self-assemble when mixed with water, only in a different mode than the soaps and detergents. Considering of the ii pendant alkyl chains present in phospholipids and the unusual mixed charges in their head groups, micelle formation is unfavorable relative to a bilayer structure. If a phospholipid is smeared over a small hole in a sparse piece of plastic immersed in water, a stable planar bilayer of phospholipid molecules is created at the hole. As shown in the post-obit diagram, the polar head groups on the faces of the bilayer contact water, and the hydrophobic alkyl bondage class a nonpolar interior. The phospholipid molecules can move about in their half the bilayer, simply in that location is a significant free energy barrier preventing migration to the other side of the bilayer.
To see an enlarged segment of a phospholipid bilayer Click Here.
This bilayer membrane structure is also found in aggregate structures called liposomes. Liposomes are microscopic vesicles consisting of an aqueous core enclosed in one or more phospholipid layers. They are formed when phospholipids are vigorously mixed with water. Unlike micelles, liposomes have both aqueous interiors and exteriors.
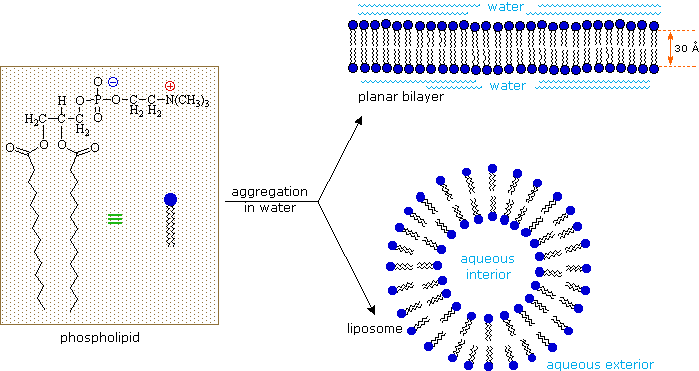
A prison cell may be considered a very complex liposome. The bilayer membrane that separates the interior of a jail cell from the surrounding fluids is largely equanimous of phospholipids, merely it incorporates many other components, such as cholesterol, that contribute to its structural integrity. Protein channels that let the transport of diverse kinds of chemical species in and out of the prison cell are likewise of import components of cell membranes. A very prissy dynamic brandish of the gramicidin channel has been created past a collaboration of Canadian, French, Castilian and US scientists, and may be examined by Clicking Here.
The interior of a cell contains a variety of structures (organelles) that conduct chemical operations vital to the cells existence. Molecules bonded to the surfaces of cells serve to identify specific cells and facilitate interaction with external chemical entities.
The sphingomyelins are likewise membrane lipids. They are the major component of the myelin sheath surrounding nervus fibers. Multiple Sclerosis is a devastating disease in which the myelin sheath is lost, causing eventual paralysis.
Prostaglandins Thromboxanes & Leukotrienes
The members of this group of structurally related natural hormones accept an extraordinary range of biological furnishings. They can lower gastric secretions, stimulate uterine contractions, lower blood pressure, influence blood clotting and induce asthma-like allergic responses. Considering their genesis in body tissues is tied to the metabolism of the essential fat acid arachadonic acrid (5,8,11,14-eicosatetraenoic acid) they are classified every bit eicosanoids. Many backdrop of the common drug aspirin upshot from its effect on the pour of reactions associated with these hormones.
The metabolic pathways past which arachidonic acid is converted to the diverse eicosanoids are circuitous and will not be discussed here. A rough outline of some of the transformations that take place is provided below. It is helpful to view arachadonic acid in the coiled conformation shown in the shaded box.
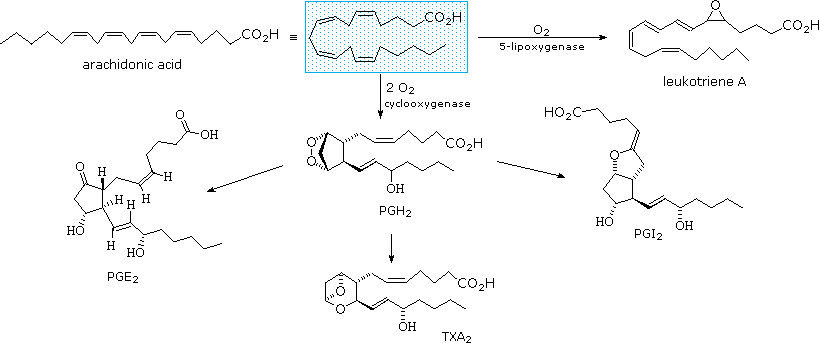
Leukotriene A is a precursor to other leukotriene derivatives by epoxide opening reactions. The prostaglandins are given systematic names that reflect their construction. The initially formed peroxide PGH2 is a common intermediate to other prostaglandins, as well every bit thromboxanes such as TXAii.
To meet a model of prostaglandin PGEii Click Hither.
Terpenes
Compounds classified as terpenes institute what is arguably the largest and most diverse class of natural products. A majority of these compounds are establish just in plants, merely some of the larger and more complex terpenes ( e.yard. squalene & lanosterol ) occur in animals. Terpenes incorporating near of the mutual functional groups are known, so this does not provide a useful means of nomenclature. Instead, the number and structural system of carbons is a definitive characteristic. Terpenes may be considered to be made up of isoprene ( more accurately isopentane ) units, an empirical feature known as the isoprene rule. Considering of this, terpenes usually have 5n carbon atoms ( n is an integer ), and are subdivided as follows:
| Nomenclature | Isoprene Units | Carbon Atoms |
|---|---|---|
| monoterpenes | ii | Cx |
| sesquiterpenes | three | C15 |
| diterpenes | 4 | C20 |
| sesterterpenes | v | C25 |
| triterpenes | 6 | Cthirty |
Isoprene itself, a C5H8 gaseous hydrocarbon, is emitted by the leaves of various plants as a natural byproduct of found metabolism. Side by side to methane it is the most mutual volatile organic compound found in the atmosphere. Examples of Cx and higher terpenes, representing the four most common classes are shown in the post-obit diagram. The initial display is of monoterpenes; larger terpenes will exist shown by clicking the "Toggle Structures" button under the diagram. Most terpenes may be structurally dissected into isopentane segments. To see how this is done click directly on the structures in the diagram.
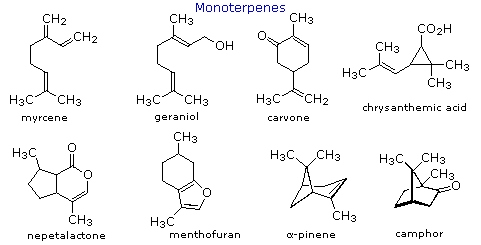
The isopentane units in most of these terpenes are easy to discern, and are defined by the shaded areas. In the case of the monoterpene camphor, the units overlap to such a degree it is easier to distinguish them past coloring the carbon chains. This is also done for alpha-pinene. In the case of the triterpene lanosterol we run into an interesting deviation from the isoprene dominion. This thirty carbon compound is conspicuously a terpene, and four of the 6 isopentane units tin be identified. However, the ten carbons in middle of the molecule cannot be dissected in this manner. Evidence exists that the two methyl groups circled in magenta and light blue have moved from their original isoprenoid locations (marked by small circles of the aforementioned color) to their present location. This rearrangement is described in the biosynthesis section. Similar alkyl group rearrangements business relationship for other terpenes that exercise not strictly follow the isoprene rule.
To see a model of the monoterpene camphor Click Here .
Polymeric isoprenoid hydrocarbons have also been identified. Rubber is undoubtedly the best known and most widely used compound of this kind. Information technology occurs as a colloidal intermission called latex in a number of plants, ranging from the dandelion to the rubber tree (Hevea brasiliensis). Safe is a polyene, and exhibits all the expected reactions of the C=C function. Bromine, hydrogen chloride and hydrogen all add with a stoichiometry of ane molar equivalent per isoprene unit of measurement. Ozonolysis of rubber generates a mixture of levulinic acrid ( CH3COCHiiCH2COtwoH ) and the corresponding aldehyde. Pyrolysis of rubber produces the diene isoprene along with other products.
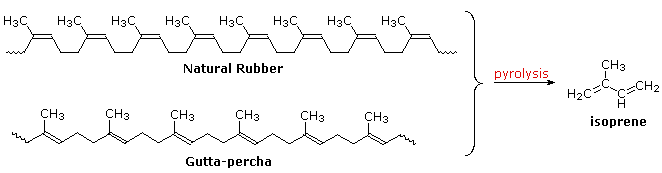
The double bonds in rubber all have a Z-configuration, which causes this macromolecule to adopt a kinked or coiled conformation. This is reflected in the concrete properties of rubber. Despite its high molecular weight (virtually 1 million), rough latex safe is a soft, sticky, elastic substance. Chemical modification of this material is normal for commercial applications. Gutta-percha (structure above) is a naturally occurring Due east-isomer of safety. Here the hydrocarbon bondage adopt a uniform zig-zag or rod similar conformation, which produces a more than rigid and tough substance. Uses of gutta-percha include electrical insulation and the roofing of golf assurance.
To see a model of the rubber chain Click Here .
Steroids
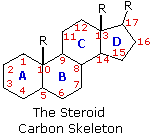
The important class of lipids called steroids are actually metabolic derivatives of terpenes, but they are customarily treated as a separate group. Steroids may be recognized past their tetracyclic skeleton, consisting of three fused 6-membered and one 5-membered ring, as shown in the diagram to the right. The four rings are designated A, B, C & D as noted, and the peculiar numbering of the ring carbon atoms (shown in cherry-red) is the result of an before misassignment of the structure. The substituents designated by R are often alkyl groups, just may as well accept functionality. The R grouping at the A:B ring fusion is most commonly methyl or hydrogen, that at the C:D fusion is usually methyl. The substituent at C-17 varies considerably, and is unremarkably larger than methyl if it is not a functional group. The most mutual locations of functional groups are C-three, C-four, C-7, C-11, C-12 & C-17. Ring A is sometimes aromatic. Since a number of tetracyclic triterpenes likewise have this tetracyclic construction, information technology cannot exist considered a unique identifier.
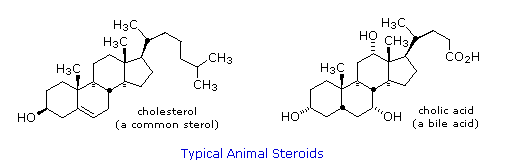
Steroids are widely distributed in animals, where they are associated with a number of physiological processes. Examples of some of import steroids are shown in the post-obit diagram. Different kinds of steroids will exist displayed by clicking the "Toggle Structures" button under the diagram. Norethindrone is a constructed steroid, all the other examples occur naturally. A common strategy in pharmaceutical chemistry is to take a natural compound, having certain desired biological properties together with undesired side effects, and to modify its construction to raise the desired characteristics and diminish the undesired. This is sometimes accomplished by trial and error.
The generic steroid structure drawn above has 7 chiral stereocenters (carbons 5, 8, nine, 10, 13, 14 & 17), which means that it may have as many every bit 128 stereoisomers. With the exception of C-5, natural steroids by and large have a single mutual configuration. This is shown in the last of the toggled displays, forth with the preferred conformations of the rings.
Chemical studies of the steroids were very important to our present understanding of the configurations and conformations of six-membered rings. Substituent groups at dissimilar sites on the tetracyclic skeleton volition have centric or equatorial orientations that are fixed considering of the rigid construction of the trans-fused rings. This fixed orientation influences chemic reactivity, largely due to the greater steric hindrance of axial groups versus their equatorial isomers. Thus an equatorial hydroxyl group is esterified more rapidly than its axial isomer.
To see a model of the steroid cholesterol Click Hither.
It is instructive to examine a uncomplicated bicyclic organization equally a model for the fused rings of the steroid molecule. Decalin, short for decahydronaphthalene, exists as cis and trans isomers at the ring fusion carbon atoms. Planar representations of these isomers are drawn at the elevation of the following diagram, with respective conformational formulas displayed underneath. The numbering shown for the band carbons follows IUPAC rules, and is different from the unusual numbering used for steroids. For purposes of discussion, the left ring is labeled A (colored blue) and the correct band B (colored red). In the conformational drawings the ring fusion and the athwart hydrogens are black.
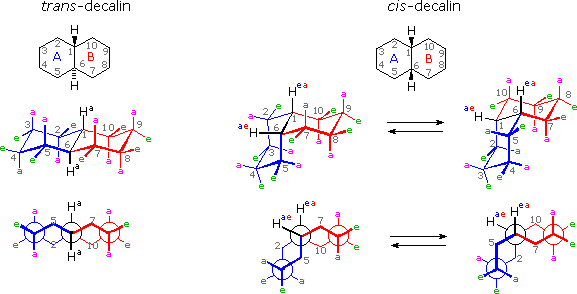
The trans-isomer is the easiest to describe because the fusion of the A & B rings creates a rigid, roughly planar, structure made upwardly of two chair conformations. Each chair is fused to the other by equatorial bonds, leaving the angular hydrogens (Ha) axial to both rings. Note that the bonds directed above the plane of the two rings alternate from axial to equatorial and dorsum if we proceed effectually the rings from C-1 to C-10 in numerical order. The bonds directed below the rings also alternating in a complementary fashion.
Conformational descriptions of cis- decalin are complicated by the fact that two energetically equivalent fusions of chair cyclohexanes are possible, and are in rapid equilibrium as the rings flip from one chair conformation to the other. In each of these all chair conformations the rings are fused by one axial and one equatorial bond, and the overall structure is bent at the band fusion. In the conformer on the left, the cerise ring (B) is attached to the blue band (A) by an axial bond to C-i and an equatorial bond to C-6 (these terms refer to ring A substituents). In the conformer on the right, the carbon bond to C-1 is equatorial and the bond to C-6 is axial. Each of the angular hydrogens (Hae or Hea) is oriented axial to one of the rings and equatorial to the other. This human relationship reverses when double band flipping converts ane cis-conformer into the other.
Cis-decalin is less stable than trans-decalin by about 2.vii kcal/mol (from heats of combustion and heats of isomerization data). This is due to steric crowding (hindrance) of the axial hydrogens in the concave region of both cis-conformers, every bit may exist seen in the model display activated by the following button. This difference is roughly iii times the free energy of a gauche butane conformer relative to its anti conformer. Indeed three gauche butane interactions may be identified in each of the cis-decalin conformations, every bit volition exist displayed by clicking on the above conformational diagram. These gauche interactions are too shown in the model.
To see models of cis & trans-decalin Click Here.
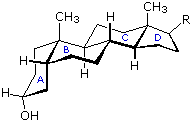
Steroids in which rings A and B are fused cis, such every bit the example on the right, practice not have the aforementioned conformational mobility exhibited by cis-decalin. The fusion of band C to ring B in a trans configuration prevents ring B from undergoing a conformational flip to another chair grade. If this were to occur, ring C would have to be fastened to ring B by two adjacent axial bonds directed 180º apart. This is too great a altitude to be bridged past the four carbon atoms making upwardly ring C. Consequently, the steroid molecule is locked in the all chair conformation shown hither. Of class, all these steroids and decalins may have i or more than six-membered rings in a boat conformation. However the loftier energy of boat conformers relative to chairs would brand such structures pocket-size components in the overall ensemble of conformations available to these molecules.
Lipid Soluble Vitamins
The essential dietary substances called vitamins are commonly classified as "water soluble" or "fat soluble". Water soluble vitamins, such equally vitamin C, are quickly eliminated from the trunk and their dietary levels need to be relatively high. The recommended daily allotment (RDA) of vitamin C is 100 mg, and amounts every bit large as 2 to 3 g are taken by many people without adverse furnishings. The lipid soluble vitamins, shown in the diagram below, are not as easily eliminated and may accrue to toxic levels if consumed in big quantity. The RDA for these vitamins are:
Vitamin A 800 μg ( upper limit ca. 3000 μg)
Vitamin D 5 to 10 μg ( upper limit ca. 2000 μg)
Vitamin Eastward 15 mg ( upper limit ca. 1 g)
Vitamin K 110 μg ( upper limit non specified)
From this information it is clear that vitamins A and D, while essential to good health in proper amounts, can exist very toxic. Vitamin D, for case, is used as a rat poisonous substance, and in equal weight is more than than 100 times as poisonous every bit sodium cyanide.
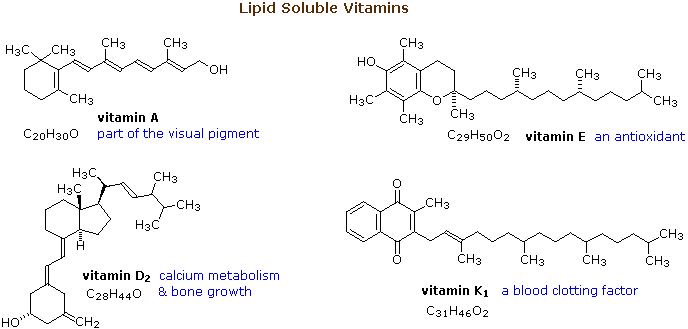
From the structures shown here, it should be clear that these compounds accept more than a solubility connection with lipids. Vitamins A is a terpene, and vitamins E and K have long terpene chains attached to an aromatic moiety. The structure of vitamin D can be described as a steroid in which ring B is cut open up and the remaining iii rings remain unchanged. The precursors of vitamins A and D have been identified equally the tetraterpene beta-carotene and the steroid ergosterol, respectively. Structures for these will be displayed by clicking on the vitamin diagram higher up .
Biosynthesis
The complex organic compounds found in living organisms on this planet originate from photosynthesis, an endothermic reductive condensation of carbon dioxide requiring light energy and the paint chlorophyll.
x CO2 + x H2O + energy  CxH2xOx + x O2
CxH2xOx + x O2
The products of photosynthesis are a class of compounds chosen carbohydrates, the well-nigh mutual and of import of which is glucose (C6H12O6). Subsequent reactions effect an oxidative cleavage of glucose to pyruvic acid (CH3COCO2H), and this in turn is transformed to the ii-carbon building block, acetate. The multitude of lipid structures described here are synthetic from acetate past enzymatic reactions that in many respects correspond to reactions used by chemists for laboratory syntheses of similar compounds. Still, an important restriction is that the reagents and conditions must be compatible with the aqueous medium, neutral pH and moderate temperatures found in living cells. Consequently, the condensation, alkylation, oxidation and reduction reactions that accomplish the biosynthesis of lipids will not make utilize of the very strong bases, alkyl halides, chromate oxidants or metal hydride reducing agents that are employed in laboratory work.
1. Condensations
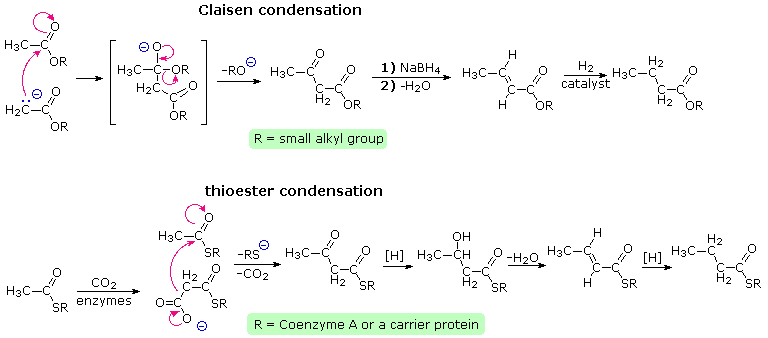
Claisen condensation of ethyl acetate (or other acetate esters) forms an acetoacetate ester, equally illustrated past the tiptop equation in the post-obit diagram. Reduction, aridity and further reduction of this product would yield an ester of butyric acrid, the overall effect existence the elongation of the acetate starting material by 2 carbons. In principle, repetition of this sequence would lead to longer chain acids, made upwards of an even number of carbon atoms. Since most of the common natural fatty acids accept even numbers of carbon atoms, this is an attractive hypothesis for their biosynthesis.
Nature's solution to conveying out a Claisen-like condensation in a living prison cell is shown in the bottom equation of the diagram. Thioesters are more reactive as acceptor reactants than are ordinary esters, and preliminary conversion of acetate to malonate increases the donor reactivity of this species. The thiol portion of the thioester is usually a protein of some kind, with efficient acetyl transport occurring by way of acetyl coenzyme A. Depending on the enzymes involved, the condensation product may exist reduced and and so further elongated so as to produce fatty acids (equally shown), or elongated by further condensations to polyketone intermediates that are precursors to a diverseness of natural phenolic compounds. Click on the diagram to meet examples of polyketone condensations.

The reduction steps (designated by [H] in the equations) and the intervening dehydrations needed for fat acid synthesis require unique coenzymes and phosphorylating reagents. The pyridine ring of nicotinamide adenine dinucleotide (NAD) and its 2'-phosphate derivative (NADP) role equally hydride acceptors, and the corresponding reduced species (NADH & NADPH) as a hydride donors. Partial structures for these important redox reagents are shown on the right. Total structures may be seen past clicking on the partial formulas.
As noted before, the hydroxyl group is a poor anionic leaving group (hydroxide anion is a strong base). Phosphorylation converts a hydroxyl group into a phosphate (POfour) or pyrophosphate (P2O7) ester, making it a much amend leaving group (the pKas at pH most 7 are 7.2 and 6.half-dozen respectively). The master biological phosphorylation reagents are phosphate derivatives of adenosine (a ribose compound). The strongest of these is the triphosphate ATP, with the diphosphate and monophosphate being less powerful. Formulas for these compounds may be seen by Clicking Here
The overall process of fatty acid synthesis is summarized for palmitic acid, CH3(CHtwo)14COiiH, in the following equation:
8 CH3CO-CoA + fourteen NADPH + fourteen H(+) + vii ATP + H2O CH3(CH2)xivCOtwoH + 8 CoA + 14 NADP(+) + 7 ADP + 7 H2PO4 (–)
CH3(CH2)xivCOtwoH + 8 CoA + 14 NADP(+) + 7 ADP + 7 H2PO4 (–)
2. Alkylations
The branched chain and cyclic structures of the terpenes and steroids are synthetic by sequential alkylation reactions of unsaturated isopentyl pyrophosphate units. Every bit depicted in the post-obit diagram, these 5-carbon reactants are fabricated from three acetate units by way of an aldol-like improver of a malonate intermediate to acetoacetate. Selective hydrolysis and reduction gives a primal intermediate called mevalonic acid. Phosphorylation and emptying of mevalonic acid so generate isopentenyl pyrophosphate, which is in equilibrium with its double bond isomer, dimethylallyl pyrophosphate. The allylic pyrophosphate group in the latter compound is reactive in enzymatically catalyzed alkylation reactions, such every bit the one fatigued in the green box. This provides support for the empirical isoprene rule.
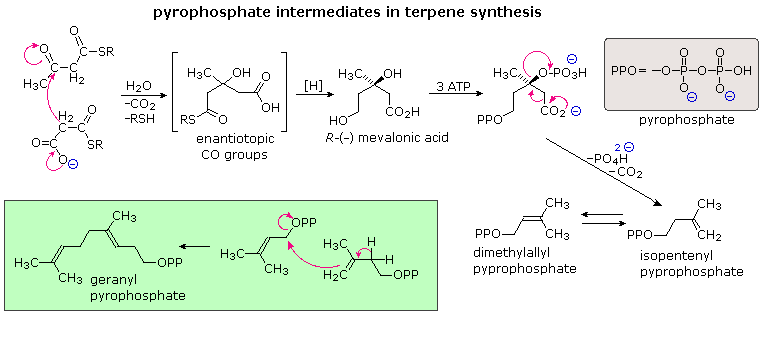
The simplest mode in which isopentane units combine is termed "caput-to-tail". This is the combination displayed in the dark-green box, and these terms are further defined in the upper equation that will appear to a higher place on clicking the "Toggle Examples" button. Not head-to-tail coupling of isopentane units is also observed, as in the chrysanthemic acid construction shown in the 2nd equation. A second click on the diagram displays the serial of cation-similar cyclizations and rearrangements, known as the Stork-Eschenmoser hypothesis, that have been identified in the biosynthesis of the triterpene lanosterol. Lanosterol is a precursor in the biosynthesis of steroids. This takes identify by metabolic removal of 3 methyl groups and degradation of the side chain.
3. An Culling Isoprenoid Synthesis
For many years, the mevalonic acid road to isopentenyl pyrophosphate was considered an exclusive biosynthetic pathway. Recently, an culling reaction sequence, starting from pyruvic acrid and glyceraldehyde-iii-phosphate, has been identified (bottom equations in the following diagram). By labeling selective carbon atoms (colored red) these distinct paths are easily distinguished. The new, DXP (1-deoxyxylulose-v-phosphate) path is widespread in microorganisms and chloroplast terpenes. The rearrangement to ii-methylerythritol-four-phosphate is an extraordinary transformation.

| Return to Tabular array of Contents |
|---|
This page is the property of William Reusch. Comments, questions and errors should exist sent to whreusch@msu.edu.
These pages are provided to the IOCD to aid in capacity building in chemical education. 05/05/2013
![]()
Lipids Are Soluble In What,
Source: https://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/lipids.htm
Posted by: jonesclumand.blogspot.com


0 Response to "Lipids Are Soluble In What"
Post a Comment